Medical Device Traceability
Streamlining Medical Device Traceability and Patient Safety
Implementation of vTag® Technology for Medical Devices
Workflow
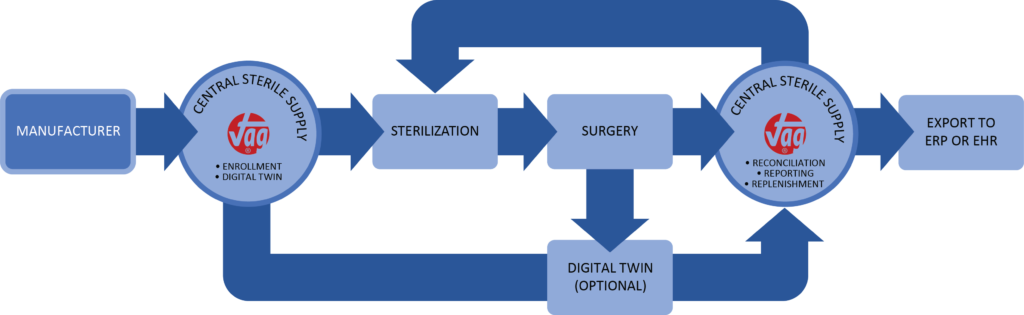
Templating and Registration
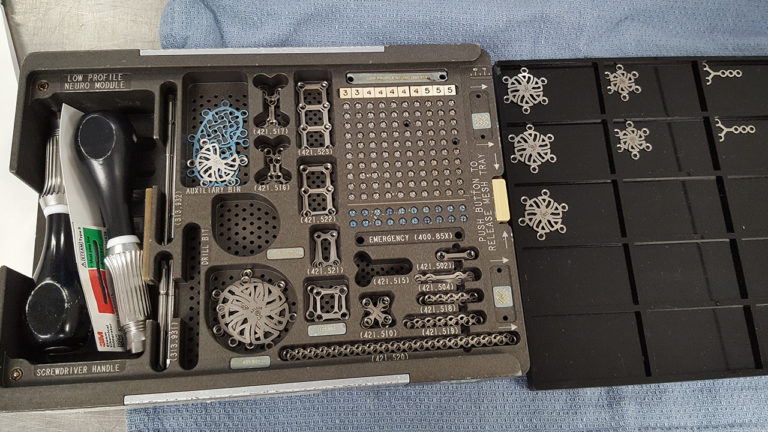
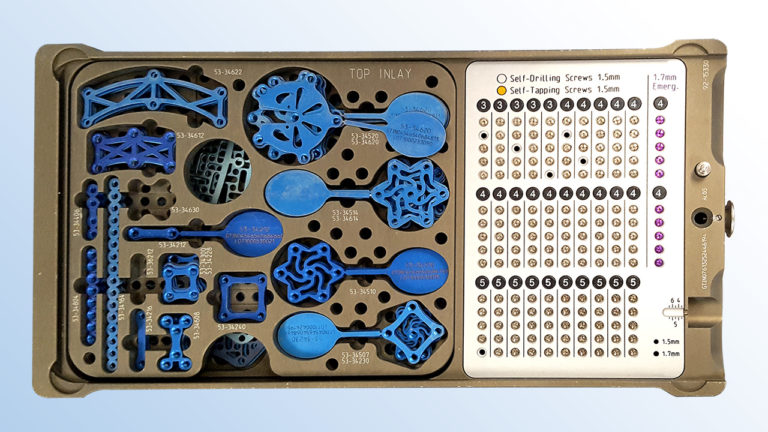
Utilizing an overhead camera that moves along a horizontal plane, our imaging system ensures screws can remain in the surgical tray during scanning. Plates and burr hole covers, which are typically stacked in the surgical tray, are briefly transferred to a generic tray to facilitate overhead scanning, ensuring visibility.


Why UDI Compliance Matters?
Meeting Stringent Requirements
US FDA UDI
The US FDA’s Final UDI regulation emphasizes the need for readily available UDI information at the point of use, eliminating the requirement for direct marking of implants.
EU MDR
According to Annex VI, Part C, 6.1.3 of the EU Medical Device Regulation (MDR), the UDI of implantable devices must be identifiable prior to implantation.
SFDA UDI Regulation
Chapter 2, 2.4 of the Saudi Food and Drug Authority (SFDA) UDI Regulation mandates that the full UDI (UDI-DI) and (UDI-PI) of an implantable device should be readily available, either electronically or scannable, at the point of implantation.
Implementation of vTag® Technology for Medical Devices
vTag® Solves for Insufficient Technologies

To meet traceability requirements, the medical device industry is implementing a range of technologies often centered on applying traditional solutions such as labels in the form of 1D/2D barcodes and RFID linked to existing ERP systems. These traditional labelling methods are well proven technologies but have fundamental drawbacks.
Current Technology Drawbacks
■ Items are too small to attach labels
■ Labels are not intrinsic to the part
■ Easily copied/removed/destroyed
■ Does not meet new regulations
■ Physical labels affect parts
■ High cost of RFID and direct part marking
Medical Device UDI Observations
Hospital systems grapple with an array of challenges when it comes to labelling consistency. From multiple barcodes on labels to varying formats and locations, the lack of uniformity poses significant obstacles for clinical staff at the point of care (POC). These inconsistencies stem from transitions occurring at manufacturers, such as version changes, mergers or acquisitions, and labelling agency conversions, which often aren’t effectively communicated to providers.
In the field, these procedural trays are replenished as needed and typically do not return to the manufacturer. This practice underscores the industry-wide challenge of maintaining consistent labelling across diverse settings and scenarios.
We recognize the critical importance of addressing these labelling inconsistencies. Our innovative solutions are designed to streamline labelling processes, ensuring clarity and efficiency at the point of care. From standardized barcode formats to comprehensive communication protocols, we empower hospital systems to overcome the challenges associated with labelling variability.

Geisinger Case Study





ABSTRACT
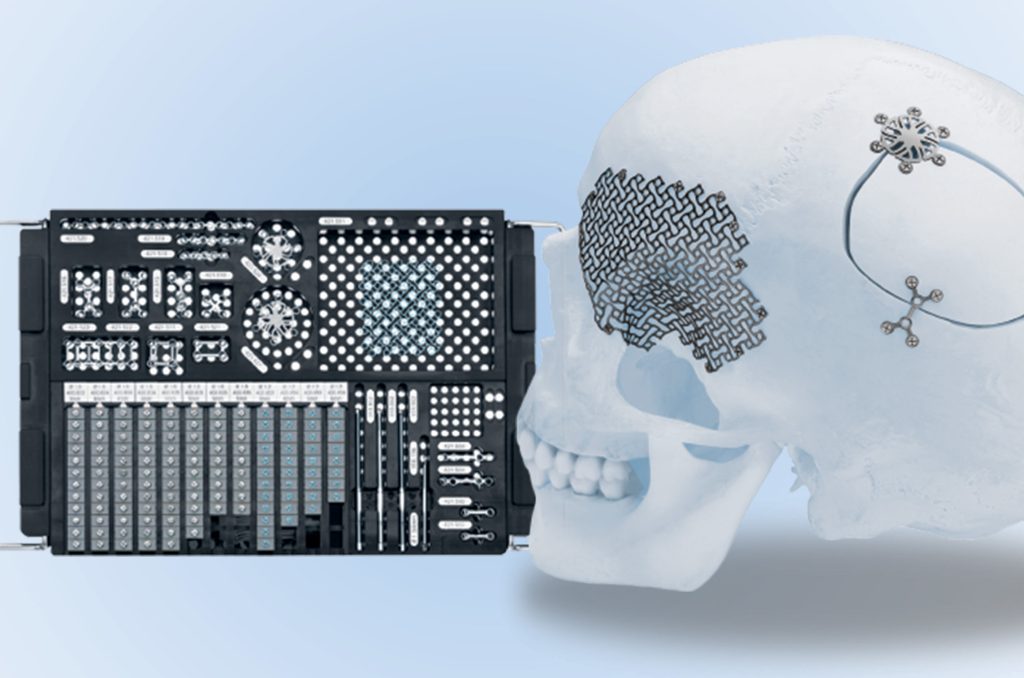
Importance: Small and mini fragment implantable medical devices (0.5mm – 4.4mm) such as plates and screws that are placed into surgical trays pose a challenge to unique device identification (UDI) tracking because they cannot be directly marked and are often separated from all labeling prior to reaching the patient at the point of care.
Objective: To demonstrate a novel optical system’s ability to track the unique identities of very small implant.
- Publication Link
Design: We performed a series of 16 simulated craniotomy surgeries involving 953 unique implants as small as 1.5mm in size, representing 13 different implants from two manufacturers, to test whether the system could accurately track all components through these simulations.
Setting: This study was performed within the central sterile supply department at a large hospital, Geisinger Medical Center, in Danville, PA, USA.
Participants: No patients or patient data were involved in this study.
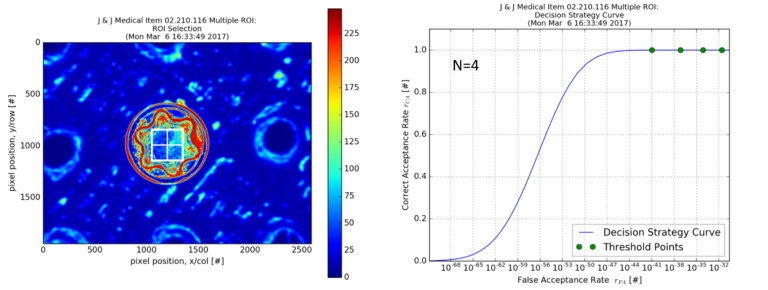
Intervention: A commercially available optical system (QuanTEK®, Covisus, Inc.) was used to rapidly capture the unique surface texture of implants at a micrometer scale and convert this to an intrinsic, immutable “fingerprint” (vTag®) for each item. The UDI information was associated with the generated vTag® when implants are placed in a surgical tray. Surgical trays of these implants were then subjected to a series of 16 simulated surgeries and after each surgery, implants remaining in the tray were scanned and identified to determine which had been used, moved, or added to the trays and their UDI information was recovered at each instance.
Main Outcomes and Measures: Primary outcome measure was the percentage of implants accurately identified. Secondary outcome was a process analysis of how the solution could be implemented outside the operating room, such as the hospitals central sterile supply facility.
Study Results
# of Surgeries

Implants
53 implants, some as small as 1.5mm in size, from mini fragment sets representing 13 products from two manufacturers.
Accuracy Results
100% accuracy (95% confidence interval = 99.6% to 100%)

Time
3.5 hours to prepare calibration files, 2 hours for initial scanning to vTag® all 953 implants, average 7 minutes turn around time between simulated surgeries.
Conclusions and Relevance: Results demonstrate that under simulated conditions, the system was able to register and verify the unique identities of all implants through a series of simulated surgical procedures with 100% accuracy. This technology could therefore be used to finally fulfill the goal of providing hospitals and supply chain management a solution to tracking the full UDI of all implantable medical devices used in the operating room, including the smallest devices.
